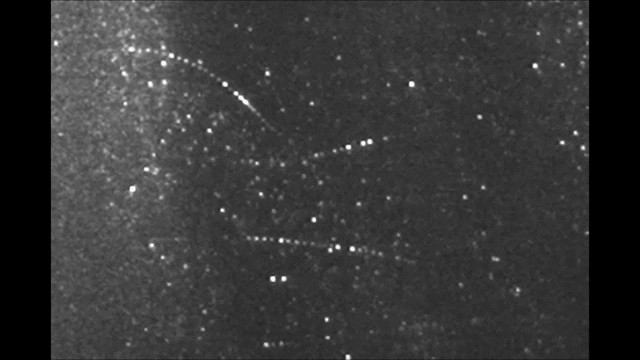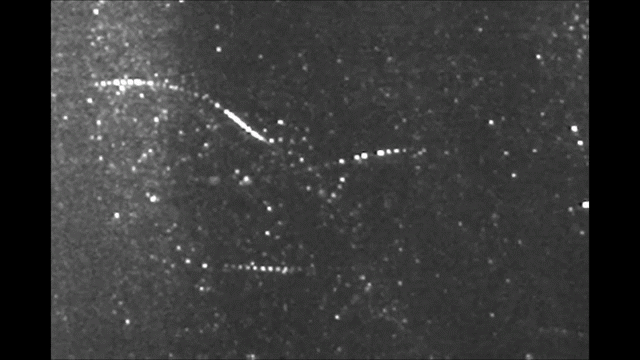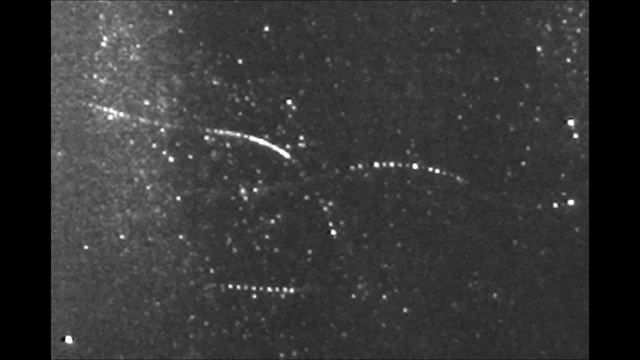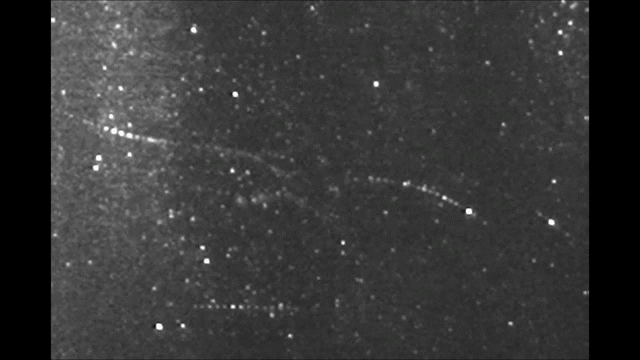
Before it was accepted that atoms are made of electrons orbiting a nucleus, Sir Walter Thomson, a British scientist famously known after his lordship title 1st Baron Kelvin, noted in an 1867 paper titled “On Vortex Atoms”, the following:
“Helmhotlz’s admirable discovery of the law of vortex motion […] inevitably suggests that Helmholt’z rings are the only true atoms. Helmholtz has provided an absolutely inalterable quality in the motion of any portion of a perfect fluid in which the peculiar motion which he calls ‘Wirbelbewegung’(i.e., voriticty) has once been created. Thus any portion of a perfect fluid which has ‘Wirbelbewegung’ has one of recommendation of Lucretius’s atoms — infinitely perennial specific quality.”
Lord Kelvin by Hubert von Herkomer
Lord Kelvin is among the most revered scientist of the late 19th century famous for his seminal work in thermodynamics, discovering the absolute zero temperature and popularizing kinematics. For over 50 years, he was Professor of Natural Philosophy at the University of Glasgow where he left an indelible impact in our understanding of the motion of fluids. At the core of his craft is a very strong hold on mathematics and an incredible talent for modeling physical phenomena.
To understand how Lord Kelvin came to think that vortices are the elemental constituents of matter, one needs to put things in context. In the second half of the 19th century, a series of experiments by English chemist John Dalton made it clear to scientists that matter is made of discrete elements, called atoms, constituting the fundamental building blocks of the universe. While direct observation with the naked eye, or any other device, was impossible at the time, researchers were looking for mathematical objects that would exhibit the properties they thought fundamental quanta of matter should have. It was the race for atom models.

 by
by 
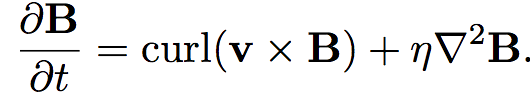
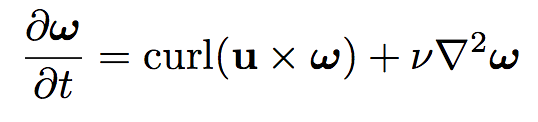 The equations dictating the evolution of vorticity and magnetic field appear to be identical, suggesting the two quantities might actually be the same.
The equations dictating the evolution of vorticity and magnetic field appear to be identical, suggesting the two quantities might actually be the same.